Using the phase diagram for h 2o what phase is water in at 1 atm
Table of Contents
Table of Contents
Are you struggling to draw a phase diagram for your chemistry class? You’re not alone. Many students find this topic to be challenging, but fear not! In this blog post, we will walk you through the process step by step so that you can confidently create your own phase diagram.
When it comes to drawing a phase diagram, students often struggle with understanding the terminology and knowing where to start. It’s easy to get overwhelmed with all the information out there. However, with a little guidance and some practice, you can master the art of drawing a phase diagram.
The first step in drawing a phase diagram is to understand what it is. A phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. These diagrams are critical in predicting phase changes and understanding the behavior of materials in various settings.
In summary, to draw a phase diagram, you need to understand the terminologies, which we will explain, know what a phase diagram is graphically, and how it predicts phase changes and material behavior.
My Experience with Drawing Phase Diagrams
As a former chemistry student, I’ve had my fair share of struggles with drawing phase diagrams. It wasn’t until I sought help from my professor and practiced regularly that I started to understand the concept. One thing that made a significant difference was breaking down the steps and practicing each section one at a time.
For example, I started with drawing the pure substance phase diagrams before progressing to mixtures. I also found it helpful to use resources online, such as videos and interactive simulations, to solidify my understanding further. Don’t be afraid to reach out for help and try different resources until you find what works for you.
The Role of Temperature and Pressure
To draw a phase diagram, you must understand the role of temperature and pressure. Essentially, phase diagrams represent the relationship between temperature, pressure, and the state of matter (or phase) that a substance is in.
Temperature impacts the behavior of atoms and molecules, causing them to move faster or slower. When atoms or molecules are moving fast enough, they overcome the attractive forces between them, and the substance changes from a liquid to a gas. On the other hand, when the temperature drops, atoms and molecules begin to slow down, and the substance changes from a gas to a liquid.
The relationship between pressure and the state of matter is also vital in drawing a phase diagram. An increase in pressure can cause a gas to liquefy, and a decrease in pressure can cause a liquid to evaporate. The pressure that a substance is exposed to is essential in determining its state of matter.
Understanding Phases
Before you start drawing a phase diagram, you must be familiar with the terminology used to describe the different phases that a substance can exist in. For example, the gaseous phase is described as a vapor, while the solid phase is described as a crystal. Additionally, a substance’s melting point is the temperature at which it transitions from a solid to a liquid, while the boiling point is the temperature at which it transitions from a liquid to a gas.
There are several phases that substances can transition through, including solid, liquid, gas, and plasma. Each of these phases exhibits unique characteristics, and understanding them is critical in drawing an accurate phase diagram.
Drawing the Phase Diagram
Now that you have a good understanding of what a phase diagram is and the terminologies involved, it’s time to start drawing. The first step is to set up your axes. The x-axis represents temperature, while the y-axis represents pressure.
Next, identify the different phases that the substance can exist in and draw lines separating them on the graph. The points where these lines intersect are called triple points, and they represent the temperature and pressure at which all three phases can coexist simultaneously.
Once you have identified and separated the different phases on your graph, you can mark the regions where each phase exists. It’s important to note that the points where lines intersect (triple points) are the only places where all three phases can coexist, while the lines themselves represent the temperatures and pressures where two phases can coexist.
Conclusion of How to Draw a Phase Diagram
Drawing a phase diagram may seem daunting at first, but with these step-by-step instructions, you should feel more confident in your ability to tackle this task. Remember to understand the terminologies, the role of temperature and pressure in determining a substance’s state of matter, and how to draw the lines that separate the different phases. With practice and persistence, you’ll have your phase diagram drawn in no time!
Question and Answer
Q. What is a phase diagram used for?
A. A phase diagram is used to represent the physical states of a substance under different conditions of temperature and pressure. They are critical in predicting phase changes and understanding the behavior of materials in various settings.
Q. What is a triple point?
A. A triple point is a point on the phase diagram where all three phases of matter (solid, liquid, and gas) coexist in equilibrium. It represents a specific temperature and pressure at which this phenomenon occurs.
Q. How is the boiling point of a substance determined?
A. The boiling point of a substance is the temperature at which it transitions from a liquid to a gas. It can be determined by heating the substance until it reaches its boiling point, at which point it will start to evaporate into a gas state.
Q. How is the melting point of a substance determined?
A. The melting point of a substance is the temperature at which it transitions from a solid to a liquid. It can be determined by heating the substance until it reaches its melting point, at which point it will begin to melt and become a liquid.
Gallery
8.1: Heating Curves And Phase Changes (Problems) - Chemistry LibreTexts
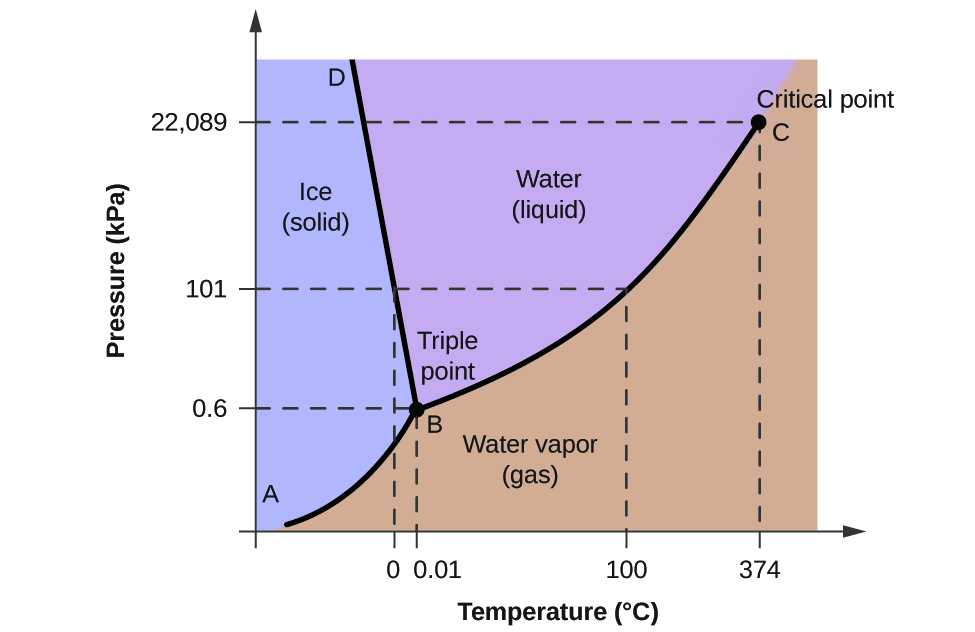
Photo Credit by: bing.com / phase pressure water temperature solid liquid chemistry diagram state gas diagrams changes heating ice equilibrium graph labeled problems curves vapor
Phase Rule
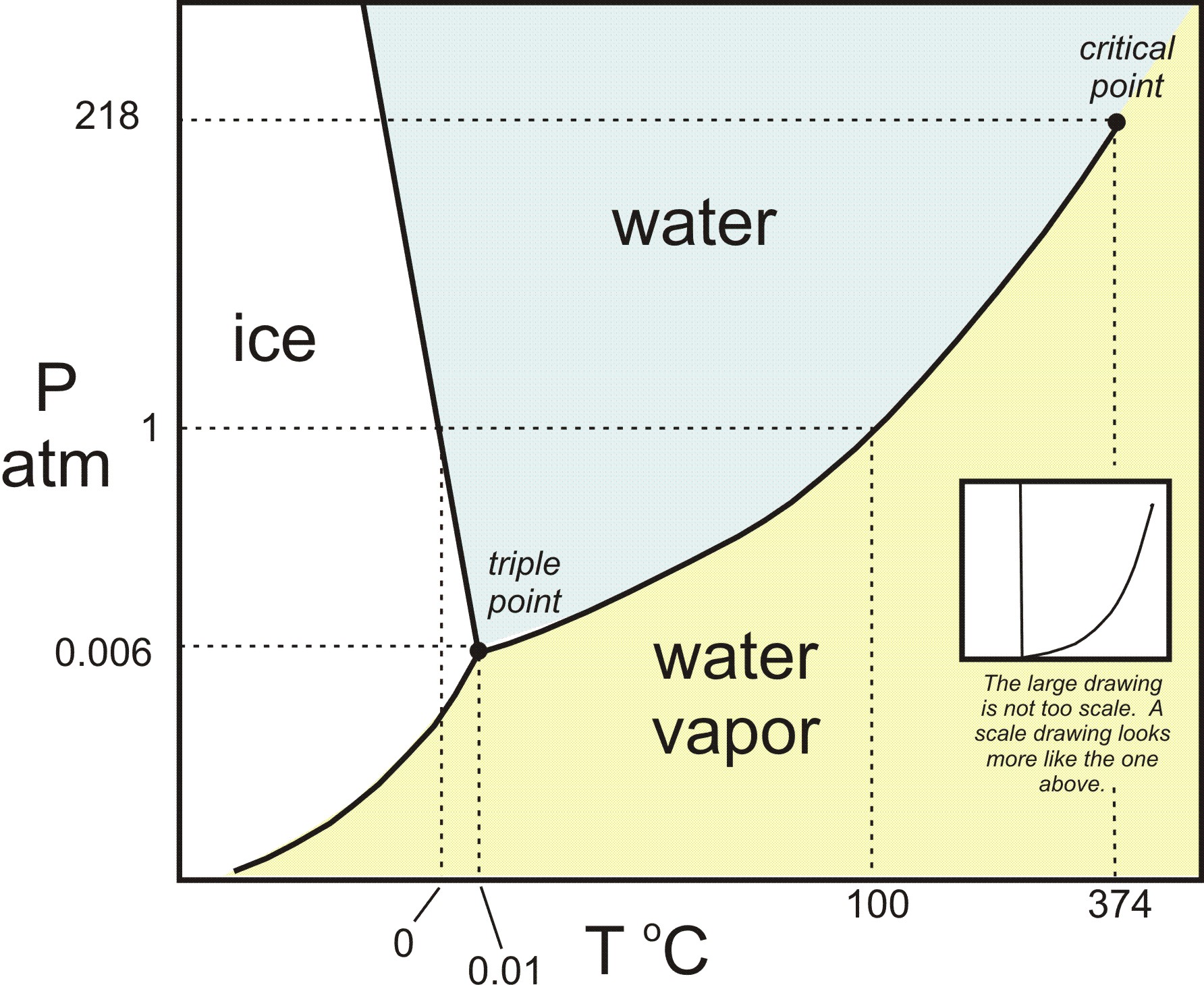
Photo Credit by: bing.com / phase diagram h2o system component rule water gibbs scale equilibria looks version h20 pressure phases point temperature liquid critical triple
How Do You Draw A Phase Diagram With A Differential Equation? | Socratic
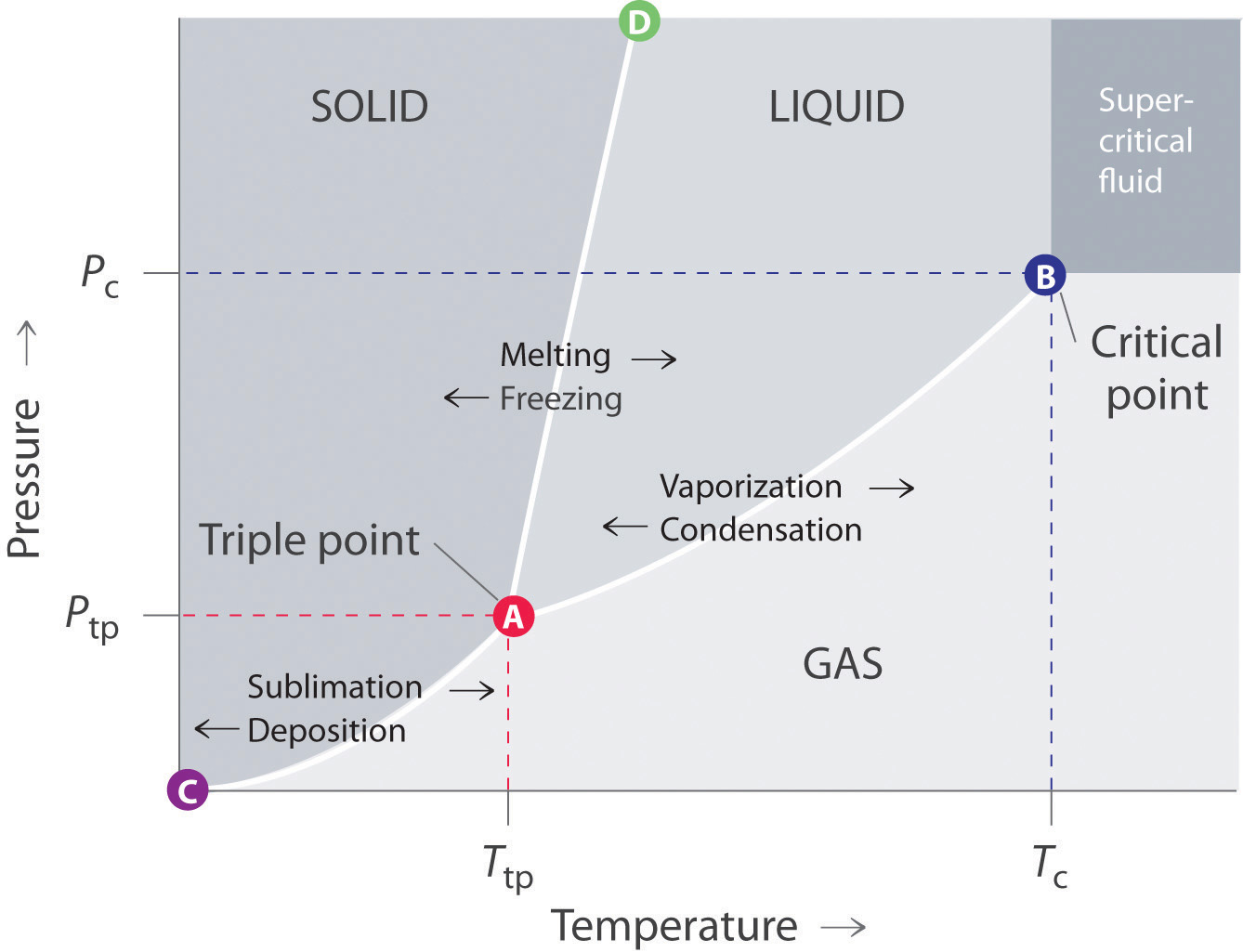
Photo Credit by: bing.com / typical substance equation differential equilibrium regions equilibria boundaries coexist chem supercritical solids curve gaseous principles
Using The Phase Diagram For H_2O, What Phase Is Water In At 1 Atm
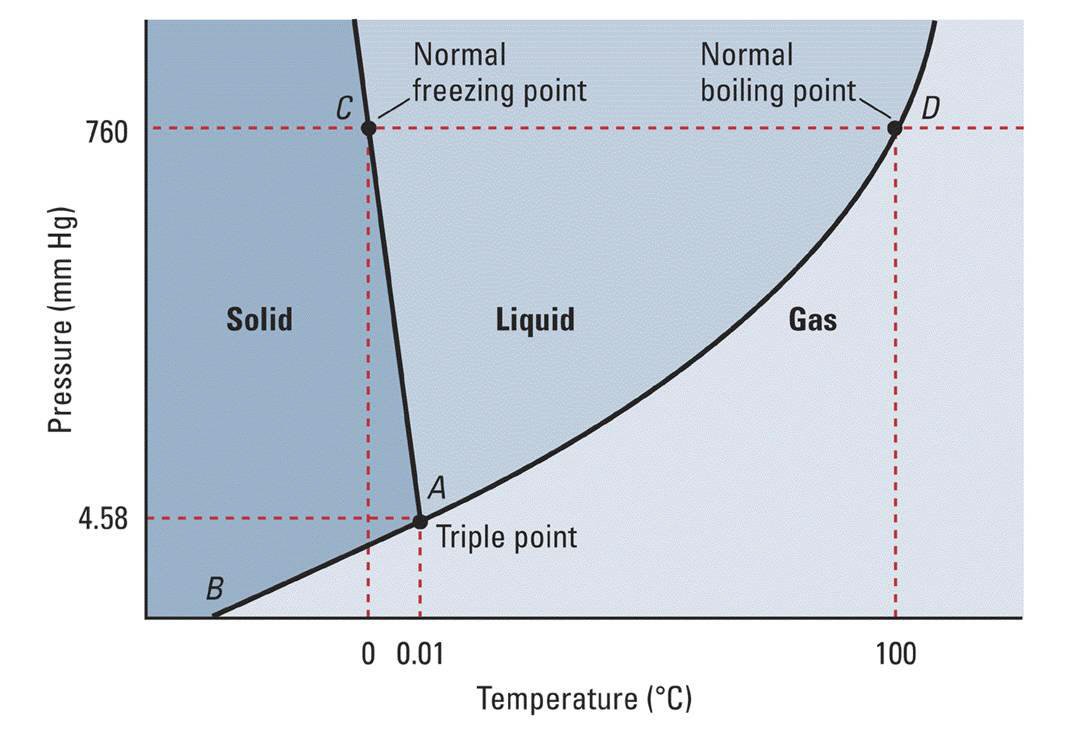
Photo Credit by: bing.com / liquid atm socratic h20 explanation 2o phases quizizz atmosphere
Download Phase_diagram.png Image From Www.periodni.com | Chemistry

Photo Credit by: bing.com / phase diagram chemistry critical point temperature gas liquid pressure solid dijagram phases transition periodni glossary above chem quality which da






